

Christian Naus
Professor Emeritus
Former Canada Research Chair in Gap Junctions in Neurological Disorders
PhD (University of Western Ontario)
MSc (University of Western Ontario)
BSc (Queen’s University)
Email: christian.naus@ubc.ca
Office: 604-827-4383,
Lab: 604-827-3436
Website: https://naus.lsi.ubc.ca/
Gap Junctions in Neural Development and Disease
Gap junctions are collections of intercellular membrane channels that join adjacent cells in every organ of the body. They allow a variety of small molecules to pass freely from cell to cell, coupling the cells metabolically and allowing them to coordinate their responses to various signals. The importance of gap junctions has become evident with the identification of congenital diseases resulting from mutations in connexin genes, including X-linked Charcot-Marie-Tooth disease, congenital cataracts, deafness, heart defects and skin diseases. In addition, reduced gap junctional coupling between cells has been detected in several cancers, and increased coupling has implications for epilepsy and stroke. Most of these disease syndromes, to greater or lesser extent, are reproduced in transgenic mice lacking specific connexins.The objective of my research program is to explore the role of gap junctions in neural development and disease, including consequences of connexin mutations on gap junction structure and function, and to explore the role of these intercellular channels in diagnosis of disease and development of novel therapeutic strategies.My research in developmental neuroscience is aimed at exploring the function of gap junctional coupling in the developing brain, using pharmacological manipulation as well as genetically modified mice designed to express normal and mutant connexin genes with specific temporal and spatial expression patterns. The role of gap junctions in the etiology and possible therapy of neurological disorders is being examined in animal models and clinical tissues related to stroke, epilepsy and brain cancer. In the area of cell biology and cancer research, we have shown that tumour cells engineered to re-establish gap junctional communication show suppression in growth and tumorigenesis. A major focus of ongoing research is aimed at determining the mechanisms underlying this tumour-suppressive effect, using genomics approaches to identify some of the links between gap junctions and expression of growth control genes. We are also exploring the repertoire of endogenous molecules which pass through gap junction channels, some of which are likely to be involved in the control of cell growth and differentiation. Given the evidence that some tumour therapeutic agents readily pass through gap junctions to enhance tumour cell killing, this research is particularly relevant to the development of novel cancer therapies.
- Freitas-Andrade, M., J. She, J. Bechberger, C.C. Naus, W.C. Sin. (2018). Acute connexin43 temporal and spatial expression in response to ischemic stroke. J Cell Communication and Signaling 12:193-204.
- Mesnil, M. T. Aasen, J. Boucher, A. Chépied , L. Cronier, N. Defamie, P. Kameritsch, D. Laird, P. Lampe, J. Lathia, E. Leithe, P. Mehta, A. Monvoisin, K. Pogoda, W.C. Sin, A. Tabernero, H. Yamasaki, E. Yeh, M.-L. Zaidan-Dagli and C.C. Naus. (2018). An update on minding the gap in cancer. Biochim Biophys Acta 1860:237-243.
- Wang, J.S.H., M. Freitas-Andrade, J.F. Bechberger, C.C. Naus, S.N. Whitehead, K.K.C. Yeung. (2018). MALDI MSI of intraperitoneally (IP) injected danegaptide for treatment of reperfusion injury, Rapid Communications in Mass Spectrometry 32(12):951-958.
- Giaume, C., J. Saez, L. Leybaert, W. Song and C.C. Naus. (2018). Connexins and pannexins in Alzheimer’s disease. In Special Issue of Neuroscience Letters on “Connexins and Pannexins in the Healthy and Diseased Nervous System”, in press.
- Van Pel, D.M., K. Harada, D. Song, C.C. Naus, and Wun Chey Sin. (2018). Modelling glioma invasion using 3D bioprinting and scaffold-free 3D culture. J. Cell Commun Signaling, 12:723-730.
- Grek, C., Z. Sheng, C.C. Naus, W.C. Sin, R. Gourdie and G.G. Ghatnekar. (2018). Novel approach to temozolomide resistance in glioblastoma: Connexin43 directed therapeutics. Current Opinion in Pharmacology 41:79-88.
- Y. Kajiwara, E. Wang, M. Wang, W.C Sin, K. Brenand, E. Schadt, C.C. Naus, J. Buxbaum, B. Zhang. (2018). Functional validation of GJA1 as a master regulator of pathogenesis in Alzheimer’s disease, Acta Neuropathologica Communications, in press.
- Leybaert, L., P. Lampe, S. Dhein, B. Kwak, P. Ferdinandy, E. Beyer, D.W. Laird, C.C. Naus, C.R. Green, R. Schulz. (2017). Connexins in cardiovascular and neurovascular health and disease: pharmacological implications. Pharmacological Review 69:396–478.
- Charveriat, M., C. C. Naus, L. Leybaert, J.C. Saez and C. Giaume. (2017). Neuroglial networking as a new therapeutic target. Frontiers in Cellular Neuroscience, Jun 26;11:174. doi: 10.3389/fncel.2017.00174.
- Laird, D.W., C.C. Naus and P. Lampe. (2017). Snapshot: Connexin gap junction proteins and linked diseases. Cell 170:1260-1260.
- Delmar, M., D. Laird, C. Naus, M.S. Nielson, V. Verselis and T. White. (2017). Gap junctions in disease, in Additional Perspectives on Cell–Cell Junctions, Editors: Carien M. Niessen and Alpha S. Yap. Cold Spring Harbour Laboratory Press, http://cshperspectives.cshlp.org/.
- Belousov, A., J. Fontes, M. Freitas-Andrade and C.C. Naus. (2017). Gap junctions and hemichannels: communicating cell death in neurodevelopment and disease. BMC Cell Biology, 18(Suppl 1):4.
- Freitas-Andrade, M., J.F. Bechberger, B.A. MacVicar, V. Viau, C.C. Naus. (2017). Pannexin1 knockout and blockade reduces neuronal ischemic injury in female, but not in male mice. Oncotarget 8:36973-3698.
- Allison, P., J. Gray, C., L. Nasmith, C.C. Naus, S. Nelson, and C. Whiteside. (2017). Creating a healthier Canada: Investing wiserly in our future. In Reflections of Canada: Illuminating Our Opportunities and Challenges at 150+ Years, pp. 57 – 62. Peter Wall Institute for Advanced Studies, UBC Press.
- Freitas-Andrade, M. and C.C. Naus. (2016) Astrocytes in neuroprotection and neurodegeneration: the role of Connexin43 and Pannexin1. Neuroscience 323:207-221. [Epub ahead of print Apr 23, 2015]
- Naus, C.C., Q. Aftab and W.C. Sin. (2016). Common mechanisms linking connexin43 to neural progenitor cell migration and glioma invasion. Seminars in Cell and Developmental Biology, 50:59-66. [Epub ahead of print Dec 17, 2015]
- Laird, D.W. and C.C. Naus. (2016). Editorial – Gerald M. Kidder bridging the gap in cell and developmental biology. Seminars in Cell and Developmental Biology, 50:1-3. [Epub ahead of print Dec 17, 2015]
- Morel, S. C. Christoffersen, M.S. Nielsen, F. Montecucco, V. Rochemont, M. Frias, F. Mach, R.W. James, C.C. Naus, M. Chanson, P.D. Lampe, L.B. Nielsen, B.R. Kwak. (2016). Sphingosine-1-Phosphate improves the outcome of cardiac ischemia/reperfusion by phosphorylating the gap junction protein Connexin43, Cardiovascular Research 109:385-96. [Epub ahead of print Jan 13]
- Sin, W.C., Q. Aftab, J. Bechberger, J. Leung, H. Chen and C.C. Naus. (2016). Astrocytes promote glioma invasion via the gap junction protein connexin43. Oncogene 35:1504-16. [Epub ahead of print Jul 13, 2015].
- Bond, S.R., J. Abramyan, K. Fu, C.C. Naus and J.M. Richman. (2016). Pannexin 3 is required for late stage bone growth but not for initiation of ossification in avian embryo. Developmental Dynamics, 245:913-924. [Epud ahead of print June 13].
- Aasen, T., M. Mesnil, C.C. Naus, P.D. Lampe and D.W. Laird. (2016). Gap Junctions and Cancer: Communicating for 50 Years. Nature Reviews Cancer 16:775-799. [Epud ahead of print Oct 21].
- Gago-Fuentes, R., J.F. Bechberger, M. Varela-Eirin, A. Varela-Vazquez, F.J. Blanco, B. Acea, E. Fonseca, C.C. Naus and Maria D. Mayan. (2016). The C-terminal domain of connexin43 modulates cartilage structure via chondrocyte phenotypic changes. Oncotarget 7:73055-73067. [Epub ahead of print Sept 22]
- Naus, C.C. and C. Giaume. (2016). Connexins and pannexins: bridging the therapeutic gap. Journal of Translational Medicine 14:330, pages 1-8. [Epub Nov 29]
- Aftab, Q., W.C. Sin and C.C. Naus. (2015). Reduction in gap junction intercellular communication promotes glioma migration. Oncotarget 6:11447-11464. [Epub ahead of print March 19]
- Decrock E., M. De Bock, N. Wang, G. Bultynck, C. Giaume, C.C. Naus, C.R Green, L. Leybaert. (2015). Connexin and pannexin signaling pathways, an architectural blueprint for CNS physiology and pathology? Cellular and Molecular Life Sciences 72:2823-2851. [Epub ahead of print June 29]
- Stewart, M.KG., J. F. Bechberger, I. Welsh, C.C. Naus, and D.W. Laird. (2015). Cx26 knockdown predisposes the mammary gland to primary mammary tumours in a DMBA-induced mouse model of breast cancer. Oncotarget 6:37185-99. [Epub ahead of print Oct 2]
- Goodson WH, Lowe L, Carpenter DO, Gilbertson M, Manaf Ali A, Lopez de Cerain Salsamendi A, Lasfar A, Carnero A, Azqueta A, Amedei A, Charles AK, Collins AR, Ward A, Salzberg AC, Colacci A, Olsen AK, Berg A, Barclay BJ, Zhou BP, Blanco-Aparicio C, Baglole CJ, Dong C, Mondello C, Hsu CW, Naus CC, Yedjou C, Curran CS, Laird DW, Koch DC, Carlin DJ, Felsher DW, Roy D, Brown DG, Ratovitski E, Ryan EP, Corsini E, Rojas E, Moon EY, Laconi E, Marongiu F, Al-Mulla F, Chiaradonna F, Darroudi F, Martin FL, Van Schooten FJ, Goldberg GS, Wagemaker G, Nangami GN, Calaf GM, Williams G, Wolf GT, Koppen G, Brunborg G, Lyerly HK, Krishnan H, Ab Hamid H, Yasaei H, Sone H, Kondoh H, Salem HK, Hsu HY, Park HH, Koturbash I, Miousse IR, Scovassi AI, Klaunig JE, Vondracek J, Raju J, Roman J, Wise JP S, Whitfield JR, Woodrick J, Christopher JA, Ochieng J, Martinez-Leal JF, Weisz J, Kravchenko J, Sun J, Prudhomme KR, Narayanan KB, Cohen-Solal KA, Moorwood K, Gonzalez L, Soucek L, Jian L, D’Abronzo LS, Lin LT, Li L, Gulliver L, McCawley LJ, Memeo L, Vermeulen L, Leyns L, Zhang L, Valverde M, Khatami M, Romano MF, Chapellier M, Williams MA, Wade M, Manjili MH, Lleonart ME, Xia M, Gonzalez MJ, Karamouzis MV, Kirsch-Volders M, Vaccari M, Kuemmerle NB, Singh N, Cruickshanks N, Kleinstreuer N, van Larebeke N, Ahmed N, Ogunkua O, Krishnakumar PK, Vadgama P, Marignani PA, Ghosh PM, Ostrosky-Wegman P, Thompson PA, Dent P, Heneberg P, Darbre P, Sing Leung P, Nangia-Makker P, Cheng QS, Robey RB, Al-Temaimi R, Roy R, Andrade-Vieira R, Sinha RK, Mehta R, Vento R, Di Fiore R, Ponce-Cusi R, Dornetshuber-Fleiss R, Nahta R, Castellino RC, Palorini R, Abd Hamid R, Langie SA, Eltom SE, Brooks SA, Ryeom S, Wise SS, Bay SN, Harris SA, Papagerakis S, Romano S, Pavanello S, Eriksson S, Forte S, Casey SC, Luanpitpong S, Lee TJ, Otsuki T, Chen T, Massfelder T, Sanderson T, Guarnieri T, Hultman T, Dormoy V, Odero-Marah V, Sabbisetti V, Maguer-Satta V, Rathmell WK, Engstrom W, Decker WK, Bisson WH, Rojanasakul Y, Luqmani Y, Chen Z, Hu Z. (2015) Assessing the carcinogenic potential of low dose exposures to chemical mixtures in the environment: The challenge ahead. Carcinogenesis, 36(S1): S254-96. international collaboration
- Rovegno M, Soto PA, Sáez PJ, Naus CC, Sáez JC, von Bernhardi R. (2015) Connexin43 hemichannels mediate secondary cellular damage spread from the trauma zone to distal zones in astrocyte monolayers. Glia 63:1185-99. International collaboration
- Nahta R, Al-Mulla F, Al-Temaimi R, Amedei A, Andrade-Vieira R, Bay SN, Brown DG, Calaf GM, Castellino RC, Cohen-Solal KA, Colacci A, Cruickshanks N, Dent P, Di Fiore R, Forte S, Goldberg GS, Hamid RA, Krishnan H, Laird DW, Lasfar A, Marignani PA, Memeo L, Mondello C, Naus CC, Ponce-Cusi R, Raju J, Roy D, Roy R, Ryan EP, Salem HK, Scovassi AI, Singh N, Vaccari M, Vento R, Vondracek J, Wade M, Woodrick J, Bisson WH. (2015) Mechanisms of environmental chemicals that enable the cancer hallmark of evasion of growth suppression. Carcinogenesis 36:S2-18. International collaboration
- Kolar K, Freitas-Andrade M, Bechberger JF, Krishnan H, Goldberg GS, Naus CC, Sin WC. (2015) Podoplanin: A marker for reactive gliosis in gliomas and brain injury. J Neuropathol Exp Neurol 74:64-74. International collaboration
- Le Vasseur M, Lelowski J, Bechberger JF, Sin W, Naus CC*. (2014) Pannexin 2 protein expression is not restricted to the CNS. Frontiers in Cellular Neuroscience 8:
- Abudara V, Bechberger J, Freitas-Andrade M, De Bock M, Wang N, Bultynck G, Naus CC, Leybaert L, Giaume C. (2014) The connexin43 mimetic peptide Gap19 inhibits hemichannels without altering gap junctional communication in astrocytes. Frontiers in Cellular Neuroscience 8:International collaboration
- Stewart MK, Plante I, Bechberger JF, Naus CC, Laird DW. (2014) Mammary gland specific knockdown of the physiological surge in Cx26 during pregnancy retains normal mammary gland development and function. PLOS One 9: e101546. National collaboration
- Amtul Z, Whitehead SN, Keeley RJ, Bechberger J, Fisher AL, McDonald RJ, Naus CC, Munoz DG, Cechetto DF. (2015) Comorbid rat model of ischemia and β‐Amyloid toxicity: Striatal and cortical degeneration. Brain Pathology 25: 24-32.National collaboration
- Amtul Z, Nikolova S, Gao L, Keeley RJ, Bechberger JF, Fisher AL, Bartha R, Munoz DG, McDonald RJ, Naus CC, Wojtowicz JM, Hachinski V, Cechetto DF. (2014) Comorbid Aβ toxicity and stroke: hippocampal atrophy, pathology, and cognitive deficit. Neurobiol Aging 35:1605-14.National collaboration
- Machtaler S, Choi K, Dang-Lawson M, Falk L, Pournia F, Naus CC, Matsuuchi L. (2014) The role of the gap junction protein connexin43 in B lymphocyte motility and migration. FEBS Lett 588:1249-1258.
- Falk L, Dang-Lawson M, Vega JL, Pournia F, Choi K, Jang C, Naus CC, Matsuuchi L. (2014) Mutations of Cx43 that affect B cell spreading in response to BCR signaling. Biol Open 3:185-94.
- Bond S, CC Naus*. (2014) Pannexins: past and present. Frontiers in Physiology: Frontiers in Physiology: Frontiers in Membrane Physiology and Membrane Biophysics 5: 1-24.
- Simonsen K, Moerman D, Naus CC*. (2014) Gap junctions in C elegans. Frontiers in Physiology: Frontiers in Membrane Physiology and Membrane Biophysics 5: 1-6.
- Le HT, Sin WC, Lozinsky S, Bechberger J, Vega JL, Guo XQ, Saez JC, Naus CC*. (2014) Gap junction intercellular communication mediated by connexin43 in astrocytes is essential for their resistance to oxidative stress. J Biol Chem 289: 1345-54.
- Giaume C, Leybaert L, Naus CC, Saez JC. (2013) Connexin and pannexin hemichannels in brain glial cells: properties, pharmacology and roles. Frontiers in Pharmacology 4:1-17. international collaboration
- Gielen PR, Aftab Q, Ma N, Chen VC, Hong X, Lozinsky S, Naus CC, Sin WC. (2013) Connexin43 confers temozolomide resistance in human glioma cells by modulating the mitochondrial apoptosis pathway. Neuropharmacology 75: 539-48.
- Kozoriz MG, Lai S, Vega JL, Sáez JC, Sin WC, Bechberger JF, Naus CC*. (2013) Cerebral ischemic injury is enhanced in a model of oculodentodigital dysplasia. Neuropharmacology 75: 549-56.International collaboration
- Chen VC, Gouw JW, Naus CC, Foster L. (2013) Connexin Multisite Phosphorylation: Mass spectrometry-based Proteomics Fills the Gap. Biochim Biophys Acta – Biomembranes 1828: 23–34.
- Matsuuchi L, Naus CC*. (2013) Gap junction proteins on the move: Connexins, the cytoskeleton and migration. Biochim Biophys Acta – Biomembranes 1828: 94–108. [IF 3.88]. local collaboration
- Mancuso M, Leonardi S, Giardullo P, Pasquali E, Tanori M, De Stefano I, Casciati A, Naus CC, Pazzaglia S, Saran A. (2013) Oncogenic radiation abscopal effects in vivo: Interrogating mouse skin. Int J Radiat Oncol Biol Phys 86: 993-9. International collaboration
- Wang N, De Vuyst E, Ponsaerts R, Boengler K, Palacios-Prado N, Wauman J, Lai CP, De Bock M, Decrock E, Bol M, Vinken M, Rogiers V, Tavernier J, Evans WH, Naus CC, Bukauskas F, Sipidio KR, Heusch G, Schulz R, Bultynck G, Leybaert L. (2013) Selective inhibition of Cx43 hemichannels by Gap19 and its impact on myocardial ischemia/reperfusion injury. Basic Res Cardiol 108: 1-16. International collaboration
- Chen VC, Kristensen AR, Foster LJ, Naus CC*. (2012) Association of connexin43 with E3 ubiquitin ligase TRIM21 reveals a mechanism for gap junction phosphodegron control. Journal of Proteome Research 11: 6134-46.
- Theodoric N, Bechberger JF, Naus CC, Sin WC. (2012) Role of gap junction protein Connexin43 in astrogliosis induced by brain injury. PLoS One 7: e47311.
- Bond SR, Wang N, Leybeart L, Naus CC *. (2012) Pannexin 1 ohnologs in the teleost lineage. Membr. Biol. 245: 483-493. International collaboration
- Bond SR, Naus CC*. (2012) RF-cloning.org: An online tool for the design of restriction-free cloning projects. Nucleic Acid Research 40(W1):W209-W213.
- Bao BA, Lai CPK, Naus CC, Morgan JR. (2012) Pannexin1 drives multicellular aggregate compaction via a signaling cascade that upregulates cytoskeletal function. Biol. Chem. 287: 8407-8416. International collaboration
- Lamiche C, Clarhaut J, Strale PO, Crespin S, Pedretti N, Bernard FX, Naus CC, Chen VC, Foster LJ, Defamie N, Mesnil M, Debais F, Cronier L. (2012) The gap junction protein Cx43 is involved in the bone-targeted metastatic behaviour of human prostate cancer cells. Clin Exp Metastasis29:111-22. International collaboration
- Decrock E, Krysko D, Vinken M, Kaczmarek A, Crispino G, Bol M, Wang N, De Bock M, De Vuyst E, Naus CC, Rogiers V, Vandenabeele P, Erneux C, Mammano F, Bultynck G, Leybaert L. (2012) Transfer of IP3 through gap junctions is critical, but not sufficient, for the spread of apoptosis.Cell Death Differ 19:947-57. International collaboration
- Bond SR, Lau AT, Penuela S, Sampaio AV, Underhill TM, Laird DW, Naus CC*. (2011) Pannexin 3 is a novel target for Runx2, expressed by osteoblasts and mature growth plate chondrocytes. Journal of Bone and Mineral Research 26:2911-22. [IF 7.05]. national collaboration
- Machtaler S, Jang C, Choi K, Naus CC, Matsuuchi L. (2011) Expression of the gap junction protein Cx43 influences B lympohocyte adhesion and cell spreading. Cell Sci. 124:2611-2621.
- Mancuso M, Leonardi S, Pasquali E, Rebessi S, Tanori M, Giardullo P, Borra F, Pazzaglia S, Naus CC, Di Majo V, Saran A. (2011) Interplay of ATP and Connexin43 in abscopal radiation tissue damage and oncogenesis in vivo. Oncogene 30:4601-4608. International collaboration
- Orellana JA, Froger N, Ezan P, Jiang JX, Bennett MV, Naus CC, Giaume C, Sáez JC. (2011) ATP and glutamate released via astroglial connexin 43 hemichannels mediate neuronal death through activation of pannexin 1 hemichannels.J Neurochem 118:826-40. International collaboration
- Orellana JA, Shoji KF, Abudara V, Ezan P, Amigou E, Saez PJ, Jiang JX, Naus CC, Saez JC, Giaume C. (2011) Amyloid beta-induced death in cultured neurons involves glial and neuronal hemichannels. Neurosci 31:4962-4977. International collaboration
- Herrero-Gonzalez S, Gangoso E, Giaume C, Naus C, Medina J, Tabernero A. (2010) Connexin43 inhibits the oncogenic activity of c-src in C6 glioma cells. Oncogene 29:5712-5723. International collaboration
- Foger N, Retamal MA, Amigou E, Kozoriz MG, Naus CC, Saez JC, Giaume C. (2010) The activation of Cx43 hemichannels is astrocytes triggered by proinflammatory cytokines enhances NMDA-induced neurotoxicity. Cell. Neurosci 45:37-46. International collaboration
- Crespin S, Bechberger JF, Mesnil M, Naus CC, Sin WC. (2010) The carboxy-terminal tail of Connexin43 gap junction protein is sufficient to mediate cytoskeletal changes in human glioma cells. Cell. Biochem 110:589-597.
- Naus CC, Laird DW. (2010) Implications and challenges of connexin connections in cancer. Nature Reviews Cancer 10:435-441.National collaboration
- Kozoriz MG, Bechberger JF, Bechberger G, Mass K, Willecke K, Naus CC*. (2010) Removal of the C-terminus of connexin43 results in enhanced damage during stroke. Neuropath. Exp. Neurol 69:196-206. International collaboration
- Guttman JA, Lin AE, Li Y, Bechberger J, Naus CC, Vogl AW, Finlay BB. (2010) Gap junction hemichannels contribute to the generation of diarrhea during infectious enteric disease. Gut 59:218-226. Local collaboration
- Sin WC, Tse M, Planque N, Perbal B, Lampe PD, Naus CC*. (2009) Matricellular protein CCN3 regulates actin cytoskeleton reorganziation. Biol. Chem 284:29935-44. International collaboration
- Lai CPK, Bechebrger J, Naus CC*. (2009) Panx2 as a novel growth suppressor in C6 glioma cells. Oncogene 28:4402-4408.
- De Vuyst E, Doecrock E, De Bock M, Van Moorhem M, Lai C, Yamasaki H, Naus CC, Evans WH, Leybaert L. (2009) Calcium-activation and inactivation of ATP release via connexin43 hemichannels is controlled by a calmodulin-arachidonic acid-ROS/NO signaling cascade. Cell Calcium 46:176-87. International collaboration
- Van Slyke JK, Naus CC, Musil LS. (2009) Conformational maturation and post-ERmultisubunit assembly of gap junction proteins. Biol. Cell 20:2451-2463. International collaboration
- Cina C, Theis M, Willecke K, Maass K, Bechberger JF, Naus CC*. (2009) Involvement of the C-terminal of Connexin43 in neuronal migration. Neurosci 29:2009-2021. International collaboration
- Doecrock E, De Vuyst E, Van Moorhem M, Laeken L, De Bock M, Vinken M, Rogiers V, D’Herde K, Evans WH, CC Naus, Leybaert L. (2009) Connexin43 hemichannels contribute to the propagation of apoptotic cell death in a rat C6 glioma cell model. Cell Death & Differentiation 16:151-163. International collaboration.
Further publications can be found here.
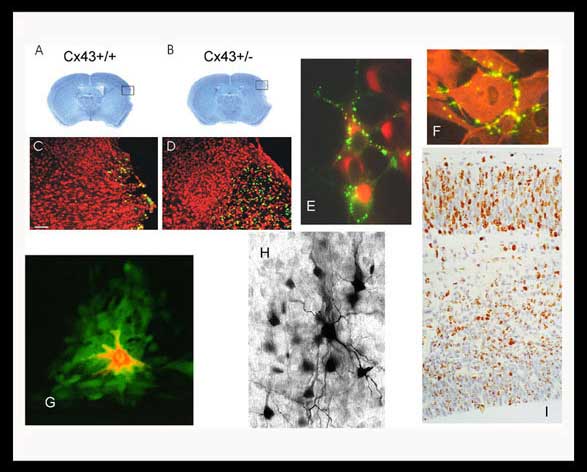
A,B,C,D: Decreased connexin43 expression results in increased infarct size and neuronal death (apoptosis green cells) following stroke injury in mice.
E: Connexin43 tagged with GFP enable us to visualize gap junction formation in live neurons.
F: Astrocytes in culture display localization of connexin43 at areas of intercellular contact.
G: A low molecular weight fluorescent dye (green) injected into a single cell (red) demonstrates the presence of gap junctions through the spread of the green dye.
H: Similar spread of a low molecular weight marker (neurobiotin) is also observed when a single neuron is injected in a brain slice.
I: The migration of new neurons can be followed as they are marked with bromodeoxyuridine in the developing cerebral cortex.